Supportive technologies for older adults is perhaps the least buzzy area of health tech. The Aging 2.0 GENerator accelerator is bucking that conventional wisdom. Its initial class of 11 early-stage companies span telecare (Lively, TTA 27 Sep), cognitive assistance (BrainAid), transportation (LiftAid) and product design (Sabi). It also connects companies to an impressive list of 75 mentors including LeadingAge/CAST, Mary Furlong Associates, the OnLok PACE community and Pfizer. Founder Katy Pike of Aging 2.0 has embedded it into San Francisco’s Institute of the Aging, which houses independent living facilities, adult day centers, and a geriatric clinic–ideal places for these startups to field test their approaches directly with their potentially 40 million 65+ market. For this the GENerator takes a not-more-than 2 percent equity stake in these companies; unlike the larger StartUp Health and Blueprint Health, it is right now too small to offer seed capital. MedCityNews
MediSafe extends med reminder tech, signs major pharma
MediSafe Project, a mobile med reminder app developed in Israel [TTA 4 Jan], is partnering with Wealth Taxi’s (!) iReminder pill caps to create a reminder system which can be used without a smartphone. The MediSafe Virtual Pillbox can use a NFC (near field communication) tag or alternatively, a Bluetooth version for web users, to create a ‘lighter’ reminder for over-the-counter or generic drugs. The more sophisticated iReminder pill caps would be used for higher-end prescription drugs. The MedCityNews article from the mHealth Summit also officially confirms Omri ‘Bob’ Shor’s ongoing discussions with New York-based AdhereTech’s [TTA 27 Aug] high-end pill reminder/dose confirmer. MediSafe is also partnering with Swiss pharmaceutical company Tillotts Pharma in “UC and ME”, a program for ulcerative colitis patients using their drug and HealthiNation on videos for Lipitor and Metformin users. While MediSafe on its own has had 170,000 downloads of its free app and ‘tens of thousands’ active (Ed.–data from Bob Shor), its real potential is in the partnerships–and the data generated by them–which now seem to be well underway.
Use of telehealth in diabetes self-management – Taiwan study
An 18-month study of diabetes patients in Taiwan has shown that using a telehealth programme was effective in enhancing blood glucose monitoring and that the patients in the programme showed improvements in glycemic control, according to a paper published this month.
Wrting in the Journal of Medical Internet Research, the authors Lichin Chen and others describe what they refer to as a diabetes telehealthcare programme whereby patients received assistance from an online diabetes self-management system to record and manage their daily activities, a 3G glucometer to monitor their glucose and a teleconsultant service to enhance their self-management activities. (more…)
Wearables on the hype cycle: a ‘Fitbit for babies’
[grow_thumb image=”https://telecareaware.com/wp-content/uploads/2013/12/3019806-poster-1280-sprouting.jpg” thumb_width=”175″ /]Is nothing sacred? Certainly not when you want a high-performing infant! FastCompany Design goes ga-ga over the Sproutling, an anklet activity monitor for the bassinet set. It tracks heart rate, skin temperature, and movement plus the room’s ambient temperature, humidity and light levels via a camera and sensors in a base station, sending data to parental smartphones. Target price not disclosed. More measurements here than our late summer baby rave, the Owlet smart sock sleep monitor which primarily alerts for dangerous baby rollover onto the stomach and trends in sleep quality, plus blood oxygen and skin temperature. There’s quite a bit in the article (more…)
mHealth Summit 2013: Sunday Venture+ Forum
Lois Drapin, Founder & CEO of The Drapin Group, provides a recap of the Venture+ Forum held the day before the official start of the mHealth Summit 2013. This is the first of her dispatches, courtesy of HIT Consultant.
[grow_thumb image=”https://telecareaware.com/wp-content/uploads/2013/12/mooc1.png” thumb_width=”150″ /]Yes, it’s true. Sunday’s Venture+ Forum, one of the day-long events that takes place before the official start of the mHealth Summit 2013, was a lot like living Gartner’s Hype Cycle in one day. Before I tell you why, let me first offer my sincere apologies to Gartner Inc. (I’ll reference the Gartner methodology in underlined italics). Absolutely no offense is meant, but this borrowed framework could be the assist I need at 1 a.m. to offer up my POV.Keynote Speaker: Jack Young, Director of Qualcomm Ventures
The day began with Jack Young, Director of Qualcomm Ventures and head of the Qualcomm Life Fund. He talked about trends that we should all know by now— the rising costs of healthcare (at $8K per human per capita, health is the most expensive subscription in our home); the aging population (a company in Japan reported that it had sold more adult diapers than baby diapers this past year). Qualcomm sees the Technology Trigger in the emergence of wearables or “mini working computers” and with big data in health such as claims data, EMR data, genomic data, consumer and social data. The wearables industry is emerging, having come into our lives connected to our smartphones. In this way, if you will, our social-ness is changing too. When you wear a wearable (watch, glasses, shoe, shirt, pin—whatever item(s) we choose), we are more likely to accept that “I’m on the journey” to health, wellness and well-being. We’re involving our friends, families and co-workers. The data that is, or will be coming from our use of wearables and other sources, will give us meaningful insights that can change behavior and health outcomes. It sounds a bit like ‘Lucy in the Sky with Diamonds’, yet who doesn’t love an investor with ‘California Dreamin’’ on his mind. I know I do.
But I already could feel the climb toward the Peak of Inflated Expectations. It really didn’t seem too far away or too high up. (more…)
Bridging ‘the neurologist gap’ in Parkinson’s via telemedicine
While Max Little’s ’30-second Parkinson’s diagnosis’ [TTA 13 Nov] is still in test, what about current patients and their treatment? Telemedicine (video consults) to the rescue! Neurologist Ray Dorsey, MD of the University of Rochester tested a ‘one free consult’ offer with a group of 55 recruited via networking site PatientsLikeMe, who were located in the five states where he is licensed to practice, using a free, secure conference platform from Vidyo. The single consult (which sounds extended or multi-part: history, neurologic examination, and recommendations) was with patients at various stages ranging from initial evaluation to third opinion. 33 of 35 consecutive patients completed the survey, reporting 90 percent satisfaction and 85 percent the same or better level of personal connection. (more…)
Motorola files patent for Emergency Alert – Neck Tattoo!
 Another pointer to the future in the world of wearable tech is this patent filed by Google-owned Motorola, for a throat ‘tattoo’, with the capability to send automatic alerts with location information to a monitoring centre or the emergency services (though the patent filing is drafted much wider!).
Another pointer to the future in the world of wearable tech is this patent filed by Google-owned Motorola, for a throat ‘tattoo’, with the capability to send automatic alerts with location information to a monitoring centre or the emergency services (though the patent filing is drafted much wider!).
The ‘tattoo’ idea isn’t as dramatic as it first appears, as the filing states it can be applied to the skin via an adhesive, or embedded in a collar or neck-band. The way it would work is by capturing the sound from a person’s throat and transmitting it to a nearby smartphone or other Mobile Communications Device (MDC). (more…)
Sony files SmartWig patent!
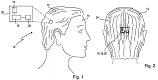 Here we have a patent filed by Sony for a sensor-laden hairpiece/wig. There are three prototypes; the Presentation Wig which has a laser pointer and allows you to change PowerPoint slides by simply pushing the sideburns – this would brighten up presentations no end ;-), the Navigation Wig which uses a GPS and vibration to direct the user, and the Sensing Wig which contains sensors to take physiological readings such as temperature and blood pressure. (more…)
Here we have a patent filed by Sony for a sensor-laden hairpiece/wig. There are three prototypes; the Presentation Wig which has a laser pointer and allows you to change PowerPoint slides by simply pushing the sideburns – this would brighten up presentations no end ;-), the Navigation Wig which uses a GPS and vibration to direct the user, and the Sensing Wig which contains sensors to take physiological readings such as temperature and blood pressure. (more…)
mHealth Summit: the warmup
News and highlights:
- Founding organizing partner mHealth Alliance is relocating from the UN Foundation in Washington, DC to Johannesburg, South Africa to be closer to its work in developing countries. Release.
- If you follow David Doherty of mHealth Insight (3G Doctor) as we do (and he does us), his mHealth/LinkedIn group will have an informal meetup at their booth #917 on Monday, 6-7:30pm. More information. He will also be speaking.
- FierceMobileHealthcare’s preview
- A spotlight on Sunday’s activities
Official Tweetstream: @mhealthsummit Hashtag: #mHealth13
Update 9 Dec: Bad weather in the area has delayed many participants or changed travel plans. MedCityNews has a timely listing of speakers’ Twitter usernames to follow here.
Telehealth & Telecare Aware is a media partner of the 2013 mHealth Summit.
Going Googly over Glass: reviews
[grow_thumb image=”https://telecareaware.com/wp-content/uploads/2013/12/3022534-inline-s-6-a-surgeons-review-of-google-glass-in-the-operating-room.jpg” thumb_width=”150″ /]Glass has been out long enough and used widely enough in the health/medical area to have some meaningful reviews. The hot area seems to be surgery, and having previewed this at the end of a minimally invasive hernia surgery during Heather Evans, MD’s talk at the NYeC Digital Health Conference [TTA 16 Nov; also her AAS article], this Editor knew more were to come. University of California-San Francisco formally received the first approval from the Institutional Review Board to use Glass during surgeries (iHealthBeat 27 Nov). Pierre Theodore, MD, a cardiothoracic surgeon at UCSF, prior to that point performed 10 to 15 surgeries with Glass assistance. From a longer article in Fast Company: “His conclusion so far: the technology is indeed useful in the operating room as an adjunct device in delivering necessary information, but it still has miles to go as a product.” Other drawbacks are its dependence on an optimal Wi-Fi signal which can be chancy in an OR, its weakness on voice commands, being able to easily scan X-rays during surgery, patient privacy and very importantly, sanitization. Completely hands-free operation is the surgeon’s goal. (Photo of Dr. Theodore courtesy of Fast Company)
Five for Friday: 3D printing
In addition to our five robots from EU Robotics Week, five medical 3D printing endeavors are spotlighted by MedCityNews: Princeton University’s artificial ear (more sensitive than human); University of Nottingham’s 3D printed bone scaffold coated with 3D printed stem cells; University of Michigan’s tiny trachea splint of biopolymers [presented at the NYeC conference on Friday]; the UK’s Open Hand Project (similar to Editor Charles’ ‘Dad, can you print me a hand?’) and from the University of Glasgow, a vision of a 3D printer to manufacture medicines. Researchers, especially those in orthopedics, are far into investigating the use of 3D printers for reconstruction and repair via the use of printed bone scaffolds. Your Editor this past week at NYC Medtech saw a short poster on a NYU School of Medicine/NYU Langone Medical Center 3D printing project involving scaffolding for bone grafts (not published); a NYU presentation on 3D scaffolding in the repair of craniofacial bone defects is here.
Previously in TTA: A real, beneficial, current use for 3D printing in medicine (Belgium) and 3D bioprinting – you may already have benefited
Your Friday ‘robot fix’
‘Blue Blazes’ indeed: wearables meet the lingerie counter
mHealth Summit: correspondents please
The inevitable: class action lawsuit against 23andMe
Breaking News (which is No Big Surprise)
Updated 6 December: 23andMe continues to sell kits, but will provide only minimal genomic information and has suspended advertising. The Washington Post reports that “In a statement Thursday night, the firm went a step further, announcing that it would only provide ancestry information and raw health data. A spokesperson for the company said that “interpreted results” would not be included.” 23andMe had earlier this week suspended advertising (Cnet/Reuters). Customers who had purchased kits prior to the FDA letter on 22 November will receive full reports. 23andMe statement on website. Matthew Herper in Forbes today on how both parties were in a ‘kind of detente’ until the bolder claims (and undoubtedly the adverts with those claims) started. And for weekend consideration, the CoreGenomics (UK) blog looks at the genomic truth-or-consequences.
The Class Action Lawsuit: Gigaom reports that a California plaintiff, Lisa Casey, filed on 27 November against 23andMe in the US District Court, Southern California citing false and misleading advertising of the Personal Genome Service (PGS) without “‘analytical or clinical validation” and that 23andMe “advertises and markets PGS as a reliable health aid”. Included in the class action complaint is (of course) every online and advertising claim regarding diseases and conditions that 23andMe’s genetic testing can assist a consumer, that (of course) FDA has not approved the marketing of the kits and that 23andMe markets the information to others even though “the test results are meaningless”. The lawsuit seeks damages extending well beyond the cost of the kit (of course), a jury trial and importantly to certify the class action and the attorney’s representation of same. Most interesting is the seemingly modest representation of Ms. Casey, Mark Ankcorn of his eponymous law firm (see his blog entry here). However, in tracing back his email domain, this Editor discovered that Mr. Ankcorn, a trial lawyer with a major-league track record of wins, recently joined the high-powered CaseyGerry firm of San Diego, which specializes in high-profile personal injury/death class action lawsuits including litigation against the NFL on TBI and CTE plus the 2011 Reno Air Races crash.
Our readers should not be surprised as our article last week was blunt on the red carpet 23andMe was figuratively rolling out for the lawyers: Get what your product does (your implied warrant of service) rock solid (23andMe is not at this point) and backed up by studies. Structure your claims as if a trial lawyer will come after you, because they come with the territory.
Well, they are here, and the mystery remains why 23andMe has chosen a path that for most early-stage companies would be corporate annihilation. 23andMe hit with class action over “misleading” genetic ads Filing (PDF) Hat tip to David E. Albert, MD of AliveCor via Twitter (@DrDave01)
Related articles of interest: Dan Munro in Forbes, writing at the same time as this Editor on the kerfuffle, analyzes far more than here on the ‘test results are meaningless’ point. My comment is below his article (expand comments). See also Mr. Munro’s comment on Illumina (which 23andMe uses for its testing–and just gained premarket clearance for their MiSeqDx test) also confirming that 23andMe lost its Chief Legal Officer in July without a replacement, which would tend to cramp dealings with FDA. See also Bernard Munos’ ‘fumbling gene’ takedown, from a scientific POV, of same. KPBS (San Diego) coverage.
Previously in TTA: FDA tells 23andMe genomic test to stop marketing
Misfit Shine goes ‘Droid, at last
Monday’s big news in the wearable sensor world was that the 10p/US quarter-sized Misfit Shine is out in an Android version, as promised back in their distant Indiegogo days before the Khosla and Founders Fund VCs discovered it. Delayed at the end of May, and reset for mid-July [TTA 30 May] then for early 2014, the Shine is now a bright spot at places like Best Buy and Target at prices from $99 to $120, though it only works on Android 4.3 or later devices and TechCrunch is reporting that early reviewers have found it crash-prone. VentureBeat raves that the Shine now has what Nike FuelBand does not–Android. It’s also far more wearable; it now comes online in the hot new ‘champagne’ and azure colors even if the initially touted jewelry-like concepts have yet to materialize.
Update 4 Dec (Breaking News): According to TechCrunch, Misfit just raised $15.2 million from Hong Kong-based Horizons Ventures. Current investors participated in the round. This now leads to a total funding of $23 million according to CrunchBase. According to AllThingsD, founder/CEO SonnyVu claims they are on track to ship 200,000 units by end of 2013 and that the funding will go to other areas of wearable sensing such as wearable feedback, identity and payments technology as well as wearable controls and gaming. Also Mobihealthnews. Are we seeing here another hype curve?
For more on wearables, AllThingsD spotlights clothing: Athos’ workout gear, Notch’s snap-on sensors and clothing, Push’s strap for weightlifters, Heapsylon’s heart-rate monitoring t-shirts and sports bras. And HIT Consultant has a nifty infographic on the future of wearable technology in healthcare which includes the new Reebok Checklight (previewed at CES Unveiled last month) and Push. (hat tip to reader Luca Sergio via Twitter @lmsergio).







Most Recent Comments